Theme: An Insight into Innovative Approaches in Global Clinical Research and Clinical Trials
Clinical Trials 2019
- About Conference
- Past Conference Chairs / Co- chairs
- Clinical Trials 2019
- Sessions / Tracks
- Past Conference Report
- Keynote Speakers
- Why to Attend
- Market Anaylsis
We are glad to announce the 8th International Conference on Clinical Trials to be held in Atlanta, USA during July 19-20, 2019. The Conference brings together Academicians, Researchers, Doctors, Principle Investigators, Clinical research sites, CROs, CMOs, Investors, and senior executives from Biotech, Biopharma, Biomedical, Medical devices and Pharmaceutical industries around the globe to discuss, reflect on and develop their ideas. It offers many opportunities for professional contact and development, and is a great networking event.
Clinical Trials 2019 is a two-day program which includes thought inspiring keynote presentations, plenary talks, poster presentations, panel discussions, workshops, symposiums, special sessions and career development programs. This will discuss most recent techniques, developments, novel strategies and various disciplines involved in drug discovery, clinical research, patient centricity, clinical site & supply management, medical imaging, data management and outsourcing in clinical trials. It will educate healthcare and clinical researcher professionals about design, operation, organizing, research computing, regulatory aspects and reporting of clinical trials. It also promotes better understanding by the general public about the importance of clinical trials in prevention, diagnosis and treatment of diseases.
The conference will also witness professionals gathering from Pharmaceutical industry Research and Development wings, API: Active Pharmaceutical Ingredients industry, Drug discovery laboratories, Formulations and NDDS domains. Also executives and eminent research personalities from the departments of QC & QA: Quality control and Quality assurance, Pharmaceutical Packaging and Logistics, Clinical trials and Pharmacovigilance, Pharmaceutical Manufacturing, Scale Up and Tech transfer, Pharmacoeconomics shall be an integral part of this conference.
Take a chance and make it count in your Professional life. Attend the Clinical Trials 2019 Conference to network with your peers, exchange expertise and experiences, and arm yourself with the latest information to take your department to the next level.
We look forward to see you personally at Atlanta, USA
It is our Pleasure to show our appreciation towards our Chairs/Co-Chairs for the Sessions, namely
- Edwin G Moore, University of Illinois, Urbana
- Panayiotis P Constantinides, Biopharmaceutical & Drug Delivery Consulting LLC, USA
- Tatsuya Takagi, Osaka University, Japan
- Arwyn T Jones, Cardiff University, UK
- Prakash V Diwan, National Institute of Pharmaceutical Education and Research, India
8thInternational Conference on Clinical Trials is scheduled to be held during July 19-20, 2019 Atlanta,USA.This Clinical Trials Conference includes a wide range of Keynote presentations, Plenary talks, Symposia, Workshops, Exhibitions, Poster presentations and Career development programs
Conference series LLC Ltd Organizes 3000+Global EventsEvery Year across USA, Europe & Asia with support from 1000 more scientific societies and Publishes 700+ Open access journals which contains over 100000 eminent personalities, reputed scientists as editorial board and organizing committee members. Theconference series website will provide you list and details about the conferences organize worldwide.
Details of Clinical Trails Conference 2019:
| Conference Name | Place | Date |
|---|---|---|
| Clinical Trails Conference 2019 | Atlanta, USA | July 19-20, 2019 |
Clinical Research & Clinical Trials: Academic Perspective:
Clinical trial is a part of clinical research that follows a regulated protocol, or plan of action. Clinical trials are primarily performed to get data on safety and efficacy of the new developed drug, this data is mandatory for further approval of the drug and to bring it into the market.
The clinical trials market has been estimated to reach USD 14.2 billion in 2016 and is projected to reach around USD 22 billion by the year 2021, growing at a CAGR (compounded annual growth rate) of 7.5%, during the forecast period 2016 to 2021. Key drivers impacting the market growth are globalization of clinical trials, development of new treatments such as personalized medicine, augmenting evolution in technology, and boosting demand for CROs to conduct clinical trials. Clinical studies can be sponsored, or funded, by pharmaceutical companies, academic medical centers, voluntary groups, and other organizations, in addition to Federal agencies such as the National Institutes of Health, the U.S. Department of Defense, and the U.S. Department of Veterans Affairs. Doctors, other health care providers, and other individuals can also sponsor clinical research.
Drug Discovery and Development:
Researchers discover new drugs through insights into a disease process that allow researchers to design a product to stop or reverse the effects of the disease. Once researchers identify a promising compound for development, they conduct experiments to gather information on how it is absorbed, distributed, metabolized, and excreted, best dosage, Side effects, how it interacts with other drugs and treatments and its effectiveness as compared with similar drugs.
Bringing one new drug to the public typically costs a pharmaceutical or biotechnology company on average more than $1 billion and takes an average of 10 to 15 years. Each drug undergoes a stringent process of discovery, development, approval and finally, public use.
Clinical Operations & Project Management:
Clinical Operations have a lot of interaction with people in a range of other departments including Clinical Science, Clinical Quality Assurance, Data Management, Biostatistics and Regulatory Affairs to ensure that the data and information needed by these other departments is delivered so they can decide if a trial has been successful. The Clinical Operations function of a company is key to the delivery of clinical trials. Without this team no Clinical Research activity could be delivered. Clinical Operations teams are responsible for designing, planning and physically running Phase I – IV clinical trials. Many larger pharmaceutical companies have also looked at setting up strategic partnerships with Clinical Research Organizations to outsource some or all of their Clinical Operations activities.
Maintain required records of study activity including case report forms, drug dispensation records, or regulatory forms. Assess eligibility of potential subjects through methods such as screening interviews, reviews of medical records, and discussions with physicians and nurses. Identify protocol problems, inform investigators of problems, or assist in problem resolution efforts such as protocol revisions.
Enrollment Planning and Patient Recruitment:
Patient recruitment and up-front enrollment planning are critical to drug development programs. Patient recruitment, if not adequately planned for, can extend your development timeline by a number of years. Retention of patients throughout the life of a clinical trial is essential in order to have complete data sets for your analysis and subsequent filings. In order to optimize both, you have to have a plan and it should take into account all stakeholders from senior management at the sponsor company and the CRO partners, to the sites and investigators and study volunteers. Cambridge Healthtech Institute’s Tenth Annual Enrollment Planning and Patient Recruitment conference will cover the topics one should consider when drafting and strategically implementing a patient recruitment plan for a clinical development program.
Patient-Centric Clinical Trials:
Generally accepted principles suggest that patient involvement should extend well beyond consideration as research subjects. Patients are key stakeholders in all aspects of trial design & execution. Patient-centric drug development also offers a huge opportunity to define meaningful outcomes from the patient perspective, as a way to ensure the needs and priorities of patient populations are reflected in research. Although efforts are made to control risks to clinical trial participants, some risk may be unavoidable because of the uncertainty inherent in clinical research involving new medical products. It's important, therefore, that people make their decision to participate in a clinical trial only after they have a full understanding of the entire process and the risks that may be involved.
Innovations in clinical study designs:
Clinical study design is the formulation of trials and experiments, as well as observational studies in medical, clinical and other types of research involving human beings. Clinical trials are typically conducted in four phases, each phase is considered as separate trial and, after completion of a phase, investigators are required to submit their data for approval from the FDA before continuing to the next phase. Types of study designs are Meta-Analysis, Systematic Review, Randomized Controlled Trial, Cohort Study, Comparative Study, Case-control Study, Cross-sectional study, Case Reports and Series, Animal Research Studies, Test-tube Lab Research
Research and Trials on Oncology and AIDS:
HIV clinical trials are research studies that look at new ways to prevent, detect, or treat HIV/AIDS. Clinical trials are the fastest way to determine if new medical approaches to HIV/AIDS are safe and effective in people. clinical trials under way include studies of new HIV medicines, studies of vaccines to prevent or treat HIV, and studies of medicines to treat infections related to HIV.
There are several types of cancer clinical trials, including treatment trials, prevention trials, screening trials, and supportive and palliative care trials. Each type of trial is designed to answer different research questions and will help researchers learn things that will help people in the future.
Clinical Trials on different Diseases:
Clinical Trials for different diseases and disorders are conducted for evaluating one or more interventions (for example, drugs, medical devices, approaches to surgery or radiation therapy) for treating a disease, syndrome, or condition and also finding ways to prevent the initial development or recurrence of a disease or condition. These can include medicines, vaccines, or lifestyle changes, among other approaches. Some examples of the diseases/disorders for which clinical trials conducting are Cardiovascular, Digestive system, Respiratory system diseases and other parasitic, viral, bacterial and fungal diseases. And Clinical Trials on behaviors, mental, sleep and eating disorders.
Medical Imaging in Clinical Research:
Medical imaging has increased exponentially in routine clinical practice. This has been reflected in a rapidly increasing use of medical imaging in clinical trials, through all phases. More recently this has culminated in a number of inter-disciplinary meetings with the various stake holders, including the FDA. Discover new technologies in medical imaging, and how to implement them in your clinical research. As the pharmaceutical, biotech and medical device industry continues to identify ways to improve and speed up product development, medical imaging plays a more significant role.
Clinical Data Management and Analytics:
Clinical data management is the process of collection, cleaning, integration and management of subject data in compliance with regulatory standards. It is a critical phase in clinical research, which leads to generation of high-quality, reliable, and statistically sound data from clinical trials, this has been facilitated by the use of software applications that maintain an audit trail and provide easy identification and resolution of data discrepancies. CDM also supports the conduct, management and analysis of studies across the spectrum of clinical research. The ultimate goal of CDM is to assure that data support conclusions drawn from research. Achieving this goal protects public health and confidence in marketed therapeutics.
Future of Clinical Trials and Technology Innovations:
Clinical study design comprises the quantity of study volunteers, their segmentation based on varying factors, and their treatment throughout the clinical trial process. Study design is a key component of clinical trials, and the treatment of all patients directly impacts the statistical validity of data. Study group assignment has also been comprehensively improved in recent years. Researchers have found many benefits to randomized assignment versus observational assignment, based on characteristics like gender, age, race, etc. The randomized method has been found to yield more reliable results than observational study assignments.
In recent years, the use of Adaptive design methods in clinical research has become increasingly popular due to its flexibility and efficiency. Adaptive designs offer the potential to reduce study duration and patient exposure whilst maximizing the probability of a successful outcome. Another innovation in clinical trials is the Bucket design. Bucket trials are designed to utilize one particular drug and test that drug against a number of different diseases. The advantage of this approach is that patients with different diseases can be 'pooled' together under one larger trial instead of lots of smaller trials, thereby saving time and resource in a similar approach, and there are more innovations in clinical trials.
CRO, Sponsorship & Outsourcing for Clinical Trials:
CRO (Contract Research Organization) is an organization that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis. Many CROs specifically provide clinical-study and clinical-trial support for drugs and/or medical devices. CROs range from large, international full-service organizations to small, niche specialty groups. A CRO may provide such services as biopharmaceutical development, biologic assay development, commercialization, preclinical research, clinical research, clinical trials management, and pharmacovigilance. CROs also support foundations, research institutions, and universities, in addition to government organizations.
Sponsorship: In the conduct of clinical trials, a sponsor is an individual (institution, company or organization) that takes the responsibility to initiate, manage or finance the clinical trial, but does not actually conduct the investigation. A sponsor-investigator, on the other hand, takes on the responsibility as a clinical study sponsor and also conducts or oversees the clinical trial. Thus, a sponsor-investigator must comply with the applicable regulatory requirements that pertain to both the sponsor and the investigator
Clinical Trial Site Selection and Management:
The conduct of clinical trials is one of the most expensive aspects of the development of new medicinal products. It is important, therefore, that the studies should produce high-quality data in the shortest possible time. More companies are trying to use single, larger, complicated trials in an attempt to gain the greatest amount of information about a product and thus reduce the lead time to market. A key element in ensuring this goal is the close cooperation between those responsible for the provision of the clinical trial supplies. The provision of clinical trial supplies is usually organized by a special group, often within the product development department, and it is prudent to discuss a proposed trial with this group at an early stage so that any potential difficulties can be identified and resolved.
The value of some study drugs can reach tens of millions of dollars, making it essential to avoid overproduction, oversupply, and inventory expiration. With the high costs and strict handling requirements for many biopharmaceutical products entering clinical development, the logistics of clinical trial supplies are more critical than ever.
Clinical Trial Forecasting, Budgeting and Contracting:
CBI’s Clinical Trial Budgeting & Forecasting brings together CROs, sites, sponsors and vendors to collaborate on best practices for ensuring accurate and effective budgeting and forecasting in clinical trials. With growing complexities and shrinking tolerance for variance between forecasted and actual budget, it’s critical that internal and external teams work together to tackle the challenges and establish efficient processes. Join your trial counterparts in May to bridge the gap between finance and operations and address each player’s role in managing deviations including timeline delays, changes in study design, outsourcing and more.
The value of some study drugs can reach tens of millions of dollars, making it essential to avoid overproduction, oversupply, and inventory expiration. With the high costs and strict handling requirements for many biopharmaceutical products entering clinical development, the logistics of clinical trial supplies are more critical than ever. Expert management begins with the clinical trial supply strategy, and ends with returns and destruction. Integration and coordination of many third-party vendors and technical systems is needed to verify that study drugs are available in sufficient quantity and quality at the various stages of clinical distribution. Clinical supplies management provides full traceability of drug supply from manufacturing to dispensation and destruction - making it a key factor in study success, as it avoids information gaps and reduces risks such as out of stock or expiration.
Globalization of Clinical Trials:
The globalization of clinical research is a relatively recent phenomenon, in which many of these studies are taking place on a global scale, with a significant increase of clinical trials in developing countries. Developed markets in the United States, Western Europe, Germany, and Japan still generate the lion’s share of clinical trial activity. Nearly 31% of the world's clinical trials are reportedly conducted outside of the United. According to the report China, Japan, India, and Korea are the most active settings for clinical trials among developing nations. It is predicted that Japan as the world’s second-largest pharmaceutical market by 2015.
According to the ClinicalTrials.gov the total number of studies registered in 2016 is 231,756. The percentage of studies registered from United States is 37%, Non-U.S is 47%. It is estimated to reach more than 280,000 study registries by 2017.
Medical and Clinical Case Reports:
A case report is a means of communicating something new that has been learnt from clinical practice. It could be about an unusual or previously unknown condition, a rare presentation or complication of a known disease, or even a new approach to managing a common condition. A case report provides the detailed report of symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports may contain a demographic profile of the patient and play major role in the field of medical research and evidenced based medicine. Moreover, case reports can serve as an early warning signal for the adverse effects of new medications, or the presentations of new and emerging diseases
Bioethics and Regulatory Compliance:
Bioethics is the study of the typically controversial ethical issues emerging from new situations and possibilities brought about by advances in medicine. It is also moral discernment as it relates to medical policy, practice, and research. Bioethicists are concerned with the ethical questions that arise in the relationships among life sciences, biotechnology, medicine, clinical research, and philosophy etc. One of the first areas addressed by modern bioethicists was that of human experimentation. The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research was initially established in 1974 to identify the basic ethical principles that should underlie the conduct of biomedical and behavioral research involving human subjects.
Clinical research ethics are the set of relevant ethics considered in the conduct of a clinical trial in the field of clinical research. It borrows from the broader fields of research ethics and medical ethics. Quality of clinical trials depends on data integrity and subject protection. Good Clinical Practice (GCP) is the universal ethical and scientific quality standard for conducting clinical trials. The GCP standard applies to all aspects of the clinical trial process.
Pharmacovigilance and Drug Safety:
The pharmacovigilance is related to collection, detection, assessment, monitoring, and prevention of adverse effects with pharmaceutical products, and it is needed in different stages of product life cycle, and the safety surveillance and risk management. Information received from patients and healthcare providers via pharmacovigilance agreements, plays a critical role in providing the data necessary for Pharmacovigilance to take place, in order to market or to test a pharmaceutical product, adverse event data must be submitted to the local drug regulatory authority. Finally pharmacovigilance is concerned with identifying the hazards associated with pharmaceutical products and with minimizing the risk of any harm that may come to patients by safety surveillance and risk management
We gratefully thank all our wonderful Keynote Speakers, Speakers, Conference Attendees, Students, Organizing Committee Members, Associations, Sponsors, Exhibitors and Media Partners for making Clinical Trials 2018 Conference the best ever!
The 7th International Conference on Clinical Trials & 12th World CADD & Drug Delivery Summit hosted by the Conference series LLC Ltd was held during September 24-26, 2018 at Chicago, USA based on the theme “An Insight into Innovative Approaches in Global Clinical Research and Clinical Trials”. Benevolent response and active participation was received from the Organizing Committee Members along with Scientists, Researchers, Students and leaders from various fields of Clinical Research & Trials, clinical research entities, medical groups, related associations, societies and also from government agencies, pharmaceutical, biomedical and medical device industries.
Conference series LLC Ltd expresses its gratitude to the conference Moderators, namely Frank Blondino, Brandon Furr and Siddhartha Chowdhury for taking up the responsibility to coordinate during the sessions. We are indebted to your support.
Similarly we also extend our appreciation towards our Chairs and Co-Chairs of the sessions for 3 days, namely Edwin G Moore, Panayiotis P Constantinides, Arwyn T Jones, Tatsuya Takagi and Prakash V Diwan.
The conference was initiated with the Honorable presence of the Keynote forum. The list includes:
- Oleg V Tcheremissine, Atrium Health, USA
- Edwin G Moore, University of Illinois, Urbana
- Panayiotis P Constantinides, Biopharmaceutical & Drug Delivery Consulting LLC, USA
- Luke S Fisher, Collaborative Drug Discovery, USA
- Arwyn T Jones, Cardiff University, UK
- Prakash V Diwan, National Institute of Pharmaceutical Education and Research, India
- Rodney Villanueva, Atrium Health, USA
- Matt Cavallo, Patient Activation Network, USA
- Tatsuya Takagi, Osaka University, Japan
- Guiyong (Nick) Song, The University of Missouri, USA
- Rahul R Panchal, Leonine Technologies Inc., USA
- Prakash V Diwan, National Institute of Pharmaceutical Education and Research, India
- Vinod Labhasetwar, Lerner Research Institute, USA
The meeting reflected various sessions, in which discussions were held on the following major scientific tracks:
- Oleg V Tcheremissine, Atrium Health, USA
- Edwin G Moore, University of Illinois, Urbana
- Panayiotis P Constantinides, Biopharmaceutical & Drug Delivery Consulting LLC, USA
- Luke S Fisher, Collaborative Drug Discovery, USA
- Arwyn T Jones, Cardiff University, UK
- Prakash V Diwan, National Institute of Pharmaceutical Education and Research, India
- Rodney Villanueva, Atrium Health, USA
- Matt Cavallo, Patient Activation Network, USA
- Tatsuya Takagi, Osaka University, Japan
- Guiyong (Nick) Song, The University of Missouri, USA
- Rahul R Panchal, Leonine Technologies Inc., USA
- Prakash V Diwan, National Institute of Pharmaceutical Education and Research, India
- Vinod Labhasetwar, Lerner Research Institute, USA
Why attend Clinical Trials 2019?
- Meet researchers from Drug discovery and Clinical Research departments
- Networking with global experts and senior executives from Pharmaceutical, Biomedical, Biopharmaceutical and Medical Devices industries
- Gain critical insights on Clinical Research
- Update yourself with latest USFDA approval policies
- Understand the Return on Investment and Pharmacoeconomics
- Meet R&D and Business Development Managers from Pharmaceutical and Biomedical Companies
Who Should Attend and Who You’ll Meet
Directors/Senior Directors/Executive Directors and Vice Presidents/Senior Vice Presidents/Executive Vice Presidents and Heads/Leaders/Partners of
- CROs and CMOs
- Clinical Research Sites
- Pharma/Biotech and Medical Device industries
- Hospitals, Associations
Medical Directors, Principal Investigators, Methodologists, and other clinical research professionals along with Clinical/Pharmaceutical and biotech industry professionals with responsibilities in:
- Clinical Research & Development
- Clinical Design/ Protocol Design/ Clinical Strategy
- Global Clinical Operations/ Clinical Outsourcing
- Biostatistics/Data management
- Patient Recruitment/Enrollment
- Clinical Trial Management/Clinical Trial Supplies
- Regulatory Affairs
Academicians:
- University Directors, Senior Professors/Assistant Professors/ Associate Professors, Research Scholars Young Researchers and Students who are working related to clinical and medical research fields
The Global Clinical Trials Market size was valued at USD 40.0 billion in 2016 and is expected to grow at a CAGR (Compound annual growth) of 5.7% over the forecast period and expected to reach USD 65.2 billion by 2025. The growth in this market is primarily driven by factors such as globalization of clinical trials, development of new treatments, evolution in technology, and increasing demand for CROs to conduct clinical trials.
Globalization of clinical trial has led to increase in investment in new product development in emerging countries thereby, having a positive impact on overall market. The availability of the vast array of services from drug discovery to post-marketing surveillance has further simplified the life for mid-size and small-scale pharmaceutical and biotechnological organizations by providing them the option to outsource what they think is beyond their core expertise.
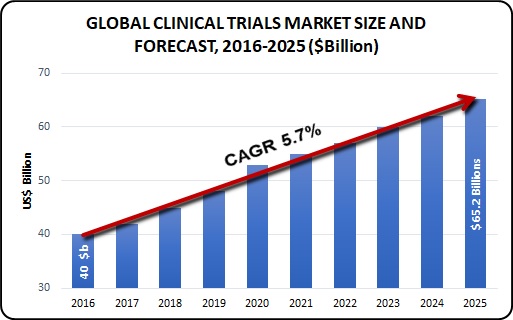
Growing prevalence of disease and incidence of new disease is expected to give further boost to the clinical trial market. Worldwide population has varied disease profile with emerging countries having the most diverse disease profile. This is expected to boost the clinical trial of new or rare disease which otherwise would not have found any sponsors. More number of patients having a specific disease would act as a stimulus for biopharmaceutical companies to invest more in clinical trials for a disease segment.
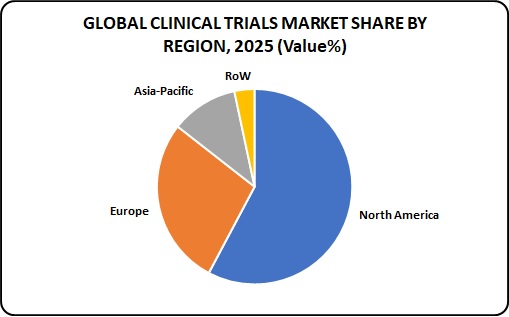
Global Clinical Trials Market share by Region:
The United States and Canada have the highest market share in the clinical trials market, followed by Europe where Germany leads the market followed by Poland and Western Europe. Asia is one of the fastest growing markets. Factors such as increasing outsourcing of clinical trials, availability of large and diverse patient population, presence of less stringent regulatory guidelines as compared to developed nations, and comparatively less cost of conducting clinical trials in Asia-Pacific countries are stimulating the growth of the market in this region.
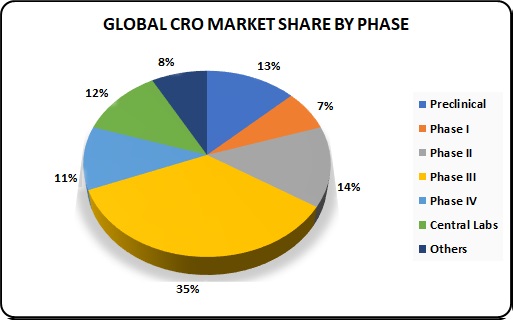
Growth trends of the Global CRO Market:
The value of the global CRO market in 2014 is estimated at about $27 billion, and it is expected to reach $32.7 billion by 2017 with CAGR of 6.6 %. A significant part of this market growth happened on the account of clinical trial phases II to IV, with their total share of the revenue by trial phase being about 60%. Medical research in Phase I accounts for as little as 7% of the generated revenue. The share of central laboratory research makes 12%, and another 13% is earned by preclinical services.
One of remarkable trends in the growth of this industry is the entry of several midsize companies into the top 20 league of multibillion revenue giants. The other notable trend refers to industry leaders’ ownership status, with more of top CROs going public. This trend indicates industry’s healthy growth and inspires mid-size companies for further development.
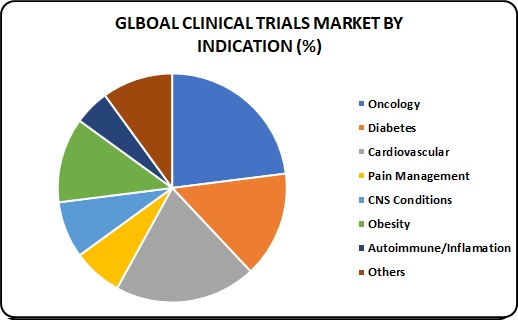
Indication Insights:
Based on indication, the oncology segment is anticipated to witness the fastest growth. According to various sources, more than USD 38.0 billion is currently spent by the healthcare industry towards preclinical and clinical development of oncology therapy products. Hence, it is anticipated to grow at a lucrative CAGR and contribute over USD 15.0 billion towards the clinical trials market by 2025.
Pain management is identified as the most lucrative segment over the forecast period owing to the increasing incidence of chronic conditions that may lead to severe pain. Furthermore, the rising investigation for new Non-Steroidal Anti-Inflammatory Drug (NSAID) and analgesic molecules is expected to be the vital impact rendering driver for this segment’s growth. In the study, pain management has been further classified into chronic and acute pain, wherein acute pain is expected to witness the fastest growth.
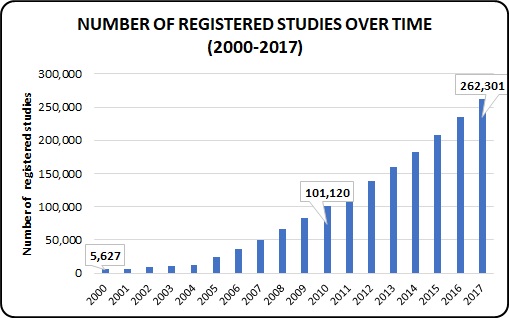
Number of Registered Studies Worldwide over Time (2000-2017):
The total number of studies registered in 2000 is 5,627 and every year it is constantly increasing the number of studies registered in 2010 is 101, 120 and finally as of December 26, 2017 the total number of studies registered worldwide is 262, 301.
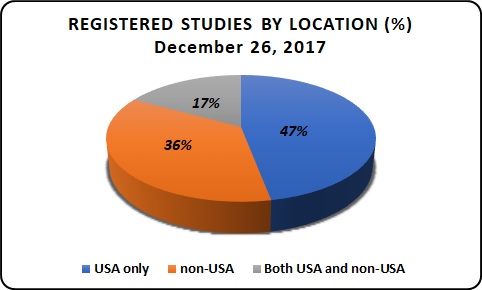
Registered Studies by Location:
As of December 26th, 2017 the total number of clinical trials studies registered worldwide is 262,301, in this 36% studies are registered in USA, 47% in non-USA and 17% in both USA and non-USA.
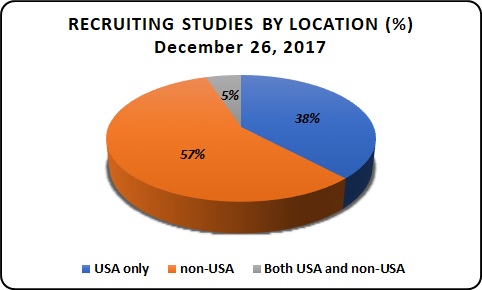
Recruiting Studies by Location:
As of December 26th, 2017 the total number of recruiting studies is 45,773, from this 57% of recruitment is in non-USA, 38% in USA and 5% recruitment in both USA and non-USA.
Conference Highlights
- Clinical Research & Clinical Trials: Academic Perspective
- Drug Discovery and Development
- Clinical Operations & Project Management
- Enrollment Planning and Patient Recruitment
- Patient-Centric Clinical Trials
- Innovations in clinical study designs
- Research and Trials on Oncology and AIDS
- Clinical Trials on different Diseases
- Medical Imaging in Clinical Research
- Clinical Data Management and Analytics
- CRO, Sponsorship & Outsourcing for Clinical Trials
- Future of Clinical Trials and Technology Innovations
- Clinical Trial Site Selection and Management
- Clinical Trial Forecasting, Budgeting and Contracting
- Clinical Supply Management
- Globalization of Clinical Trials
- Medical and Clinical Case Reports
- Bioethics and Regulatory Compliance
- Pharmacovigilance and Drug Safety
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | July 19-20, 2019 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | ||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
Abstracts will be provided with Digital Object Identifier by








