Theme: Exploring Cutting Edge Technologies and Global Clinical Trials of the Future
Clinical Trials 2016
The 2nd International Conference on Clinical Trials which is going to be held during August 22-24, 2016 at Philadelphia, USA will bring together Leading Principle Investigators, Methodologists, Clinicians, CRO’s, Biotech and Pharmaceutical industry professionals to discuss the advancements in health care therapeutics and clinical trials.
Track 1: Pre-Clinical Research
Pre-clinical research also named preclinical studies and nonclinical studies is a stage of research that begins before clinical trials, and during which important feasibility, iterative testing and drug safety data is collected. The main goals of preclinical studies are to determine the safe dose for First-in-man study and start to assess product's safety profile. Products may include new or iterated or like-kind medical devices, drugs, etc.
Related Conferences:
2nd World Congress on Pharmacology, August 08-10, 2016 Birmingham, UK; 7th Conference on Biomarkers, November 28-30, 2016 Baltimore, USA; Conference on Therapeutic Drug Monitoring , June 09-10, 2016 Dallas, USA; Preclinical and Translational Immuno-Oncology Conference, August 30-31, 2016, Boston, USA; Clinical Trials for Cancer Immunotherapy, September 1-2, 2016, Boston, USA; Summit for Clinical Ops Executives (SCOPE), January 24-26, 2017, Miami, USA; Clinical Data Strategy Conference, January 24-25, 2017 Miami, USA; Association of Clinical Research Professionals, Society for Clinical Research Sites, American Association for Cancer Research, Canadian Cancer Society, Association of Clinical Research Organizations
Track 2: Conducts of Clinical Trials
Clinical trials also known as clinical research studies that follow a pre-defined plan or protocol. Researchers design clinical trials (Clinical study designs) to answer specific research questions related to a medical product. A clinical study involves research using human volunteers (also called participants) that is intended to add to medical knowledge.
Related Conferences:
2nd Conference on Clinical Trials, August 22-24, 2016 Philadelphia, USA; 5th Conference on Translational Medicine, November 17-19, 2016 San Francisco, USA; 7th Conference on Biomarkers, November 28-30, 2016 Baltimore, USA; 4th Conference on Clinical Pharmacy, October 31-November 01, 2016 Las Vegas, USA; Clinical Trial Forecasting and Budgeting Conference, January 24-25, 2017 Miami, USA; Clinical Technology and Innovation Conference, January 25-26, 2017 Miami, USA; Managing Outsourced Clinical Trials Conference, January 25-26, 2017 Miami, USA; Leveraging Real World Data for Clinical and Observational Research Conference, January 25-26, 2017, Miami, USA; American Society of Clinical Oncology, Leukemia & Lymphoma Society, Central Society for Clinical and Translational Research, ECFS-Clinical Trial Network- Europe, Australasian Kidney Trials Network
Track 3: Clinical Study Designs
Clinical study design is the formulation of trials and experiments, as well as observational studies in medical, clinical and other types of research involving human beings. Clinical trials are typically conducted in four phases, each phase is considered as separate trial and, after completion of a phase, investigators are required to submit their data for approval from the FDA before continuing to the next phase. By taking part in clinical trials, participants can not only play a more active role in their own health care, but they can also access new treatments and help others by contributing to medical research. Types of Study Designs Meta-Analysis, Systematic Review, Randomized Controlled Trial, Cohort Study, Comparative Study, Case-control Study, Cross-sectional study, Case Reports and Series, Animal Research Studies, Test-tube Lab Research
Related Conferences:
7th Conference on Biomarkers, November 28-30, 2016 Baltimore, USA; Conference on Medical Ethics, June 09-11, 2016 London, UK; 6th Conference on Experimental Dermatology, May 05-07, 2016 Chicago, USA; 3rd Experts Meeting on Medical Case Reports, May 09-11, 2016 New Orleans, USA; 5th Clinical Trials Inspection Readiness Summit, August 08-09, 2016, Philadelphia, USA; ACRP Conference, April 29-May 2, 2017, Seattle, USA; FDA Clinical Trial Requirements Regulations, Compliance, GCP Conference, November 2-3, 2016 New Orleans, USA; Australasian Kidney, Trials Network, Chinese American Medical Society, Drug Development Institute, Indian Association for Statistics in Clinical Trials, Chinese Clinical Trial Register
Track 4: Innovations in Clinical Trials
Clinical trials for the development of new drugs are and their most part initiated and financed by industry. There are also many clinical trials initiated by academic clinical researchers. Whether initiated by industry or by academic clinical investigators clinical research is often performed in national, European and worldwide consortia, which can sometimes, be very large ones. Clinical research raises profound ethical and safety questions. The protection of participants in a clinical trial is of paramount importance. As a consequence, clinical research is highly regulated. To facilitate collaborations across borders, many aspects of this regulation are harmonized at the European level but also worldwide.
Related Conferences:
2nd World Congress on Pharmacology, August 08-10, 2016 Birmingham, UK; Conference on Therapeutic Drug Monitoring & Toxicogenomics, ,June 09-10, 2016 Dallas, USA; 10th Conference on Experimental Ophthalmology, November 28-30, 2016 Dubai, UAE; Conference on Clinical Laboratory Medicine, May 02-03, 2016 Chicago, USA; Conference on Legal Medical, June 09-11, 2016 London, UK; FDA Clinical Trial Requirements Regulations, Compliance, GCP Conference, November 2-3, 2016 New Orleans, USA; Outsourcing in Clinical Trials Nordics Conference 2016, September14-15, 2016, Copenhagen, Denmark; Mobile in Clinical Trials Conference, September 19, 2016 Boston, USA; ACCP 2016 Annual Meeting, September 25-27, 2016, Atlanta, USA; Association of Clinical Research Professionals, Society for Clinical Research Sites, American Association for Cancer Research, European Federation of Pharmaceutical Industries and Associations, American Lung Association
Track 5: Clinical Data Management and Statistics
The process of identification, analysis and either acceptance or mitigation of uncertainty in investment decision-making Essentially, risk management occurs anytime an investor or fund manager analyzes and attempts to quantify the potential for losses in an investment and then takes the appropriate action given their investment objectives and risk tolerance. Inadequate risk management can result in severe consequences for companies as well as individuals. A practice used by different companies to reduce costs by transferring portions of work to outside suppliers rather than completing it internally is called Outsourcing. It is a very important tool for reducing cost and improving quality. If an organization does one or all its work by itself, its work may affect its production quality, so an organization must realize some important areas, from which its cost is reduced and its products stay in high quality.
Related Conferences:
7th Conference on Pharmacovigilance, September 19-21, 2016 Vienna, Austria; Conference on Legal Medical, June 09-11, 2016 London, UK; 3rd European Conference on Clinical Research, October, 17-18 2016, Prague, Czech Republic; 3rd Clinical Trials Phase I and Phase IIA Summit, October 18-19, 2016, Philadelphia, USA; Clinical Trial Supply East Coast Conference 2016, October 19-20, 2016, Princeton, USA; Outsourcing in Clinical Trials Canada Conference 2016, October 19-20, 2016, Vancouver, Canada; European Federation of Pharmaceutical Industries and Associations, American Lung Association, Association of Clinical Research Professionals, Society for Clinical Research Sites
Track 6: Transforming Trial Methodologies
Clinical study design is the formulation of trials and experiments, as well as observational studies in medical, clinical and other types of research involving human beings. The goal of a clinical study is to assess the safety, efficacy, and/or the mechanism of action of an investigational medicinal product, or new drug or medical device that is in development, but potentially not yet approved by a health authority.
Design-based research is a type of research methodology commonly used by researchers in the learning sciences. Within design-based research methodology, interventions are conceptualized and then implemented iteratively in natural settings in order to test the ecological validity of dominant theory and to generate new theories and frameworks for conceptualizing learning, instruction, design processes, and educational reform.
Related Conferences:
5th Conference on Translational Medicine, November 17-19, 2016 San Francisco, USA; 10th Conference on Clinical Ophthalmology, November 28-30, 2016 Dubai, UAE; Conference on Legal Medical, June 09-11, 2016 London, UK; Clinical Practice Compliance Conference, October 23-25, 2016, Phoenix, USA; Outsourcing in Clinical Trials Southern California 2016, October 26-27, 2016, California, USA; Exploratory Clinical Development World Europe Conference 2016, October 26-27, 2016, Berlin, Germany; Clinical Development Leaders Summit, November 01-03, 2016, Shanghai, China; FDA Clinical Trial Requirements Regulations, Compliance, GCP Conference, November 02-03, 2016, New Orleans, USA; Association of Clinical Research Professionals, Society for Clinical Research Sites, American Association for Cancer Research, Canadian Cancer Society
Track 7: Clinical Trials in Oncology
Cancer is a group of more than 100 different diseases characterized by the uncontrolled, abnormal growth of cells. These cells form a lump or mass called a tumor. Cancers are divided in to types based on where it begins.
- Carcinomas: A carcinoma begins in the skin or the tissue that covers the surface of internal organs and glands. Carcinomas usually form solid tumors. They are the most common type of cancer. Examples of carcinomas include prostate cancer, breast cancer, lung cancer, and colorectal cancer.
- Sarcomas: A sarcoma begins in the tissues that support and connect the body. A sarcoma can develop in fat, muscles, nerves, tendons, joints, blood or lymph vessels, cartilage, or bone.
- Leukemia’s: Leukemia is a cancer of the blood. Leukemia begins when healthy blood cells change and grow uncontrollably. The four main types of leukemia are acute lymphocytic leukemia, chronic lymphocytic leukemia, acute myeloid leukemia, and chronic myeloid leukemia.
- Lymphomas: Lymphoma is a cancer that begins in the lymphatic system. The lymphatic system is a network of vessels and glands that help fight infection. There are two main types of lymphomas: Hodgkin lymphoma and non-Hodgkin lymphoma.
Related Conferences:
14th World Cancer Convention, November 21-23, 2016 Dubai, UAE; 14th World Congress on Cancer Therapy, December 05-07, 2016 Philadelphia, Pennsylvania, USA; 3rd World Congress on Women’s Health and Breast Cancer, October 03-05, 2016 London, UK; Conference on Legal Medical, June 09-11, 2016 London, UK; Preclinical and Translational Immuno-Oncology Conference, August 30-31, 2016, Boston, USA; Clinical Trials for Cancer Immunotherapy, September 1-2, 2016, Boston, USA; Clinical Practice Compliance Conference, October 23-25, 2016, Phoenix, USA; Outsourcing in Clinical Trials Southern California 2016, October 26-27, 2016, California, USA; Association of Clinical Research Organizations, American Society of Clinical Oncology, Association of Clinical Research Professionals, Society for Clinical Research Sites
Track 8: HIV/AIDS Clinical Trials
HIV/AIDS clinical trials are research studies done to find new ways to prevent, detect, or treat HIV/AIDS. Examples of HIV/AIDS clinical trials include studies of new HIV medicines, studies of vaccines to prevent or treat HIV, and studies of medicines to treat infections related to HIV. Clinical trials are the fastest way to determine if new medical approaches to HIV/AIDS are safe and effective in people. The benefits and possible risks of participating in an HIV/AIDS clinical trial are explained to study volunteers before they decide whether to participate in a study.
Related Conferences:
4th Conference on HIV/AIDS, STDs and STIs, October 03-05, 2016 Orlando, Florida, USA; 2nd World Congress on Pharmacology, August 08-10, 2016 Birmingham, UK; 7th Conference on Biomarkers, November 28-30, 2016 Baltimore, USA; Preclinical and Translational Immuno-Oncology Conference, August 30-31, 2016, Boston, USA; Clinical Trials for Cancer Immunotherapy, September 1-2, 2016, Boston, USA; Summit for Clinical Ops Executives (SCOPE), January 24-26, 2017, Miami, USA; Clinical Data Analytics Conference, January 24-25, 2017 Miami, USA; Association of Clinical Research Professionals, Society for Clinical Research Sites, American Association for Cancer Research, Canadian Cancer Society, Association of Clinical Research Organizations
Track 9: Diabetes Clinical Trials
Diabetes, often referred to by doctors as diabetes mellitus, describes a group of metabolic diseases in which the person has high blood glucose (blood sugar), either because insulin production is inadequate, or because the body's cells do not respond properly to insulin, or both. Patients with high blood sugar will typically experience polyuria (frequent urination), they will become increasingly thirsty (polydipsia) and hungry (polyphagia).
There are three types of diabetes:
- Type 1 diabetes
- Type 2 diabetes
- Gestational diabetes
Related Conferences:
14th Conference and Exhibition on Targeting Diabetes, October 17-19, 2016 Kuala Lumpur, Malaysia; 5th Conference on Translational Medicine, November 17-19, 2016 San Francisco, USA; 7th Conference on Biomarkers, November 28-30, 2016 Baltimore, USA; 4th Conference on Clinical Pharmacy, October 31-November 01, 2016 Las Vegas, USA; Clinical Trial Forecasting and Budgeting Conference, January 24-25, 2017 Miami, USA; Clinical Technology and Innovation Conference, January 25-26, 2017 Miami, USA; Managing Outsourced Clinical Trials Conference, January 25-26, 2017 Miami, USA; Leveraging Real World Data for Clinical and Observational Research Conference, January 25-26, 2017, Miami, USA; American Society of Clinical Oncology, Leukemia & Lymphoma Society, Central Society for Clinical and Translational Research, ECFS-Clinical Trial Network- Europe, Australasian Kidney Trials Network
Track 10: Cardiovascular Clinical Trials
Heart and blood vessel disease also called cardiovascular disease includes numerous problems, many of which are related to a process called atherosclerosis. Atherosclerosis is a condition that develops when a substance called plaque builds up in the walls of the arteries. This buildup narrows the arteries, making it harder for blood to flow through. If a blood clot forms, it can stop the blood flow. This can cause a heart attack or stroke.
There are four main types of cardiovascular diseases. They are:
- Coronary heart disease
- Stroke
- Peripheral arterial disease
- Aortic disease
Cardiovascular clinical research/trials involving new cardiovascular therapeutics and to advance the care of patients with cardiovascular diseases
Related Conferences:
6th Conference on Experimental Cardiology, November 14-16, 2015 San Francisco, USA; 7th Conference on Pharmacovigilance, September 19-21, 2016 Vienna, Austria; Conference on Legal Medical, June 09-11, 2016 London, UK; 3rd European Conference on Clinical Research, October, 17-18 2016, Prague, Czech Republic; 3rd Clinical Trials Phase I and Phase IIA Summit, October 18-19, 2016, Philadelphia, USA; Clinical Trial Supply East Coast Conference 2016, October 19-20, 2016, Princeton, USA; Outsourcing in Clinical Trials Canada Conference 2016, October 19-20, 2016, Vancouver, Canada; European Federation of Pharmaceutical Industries and Associations, American Lung Association, Association of Clinical Research Professionals, Society for Clinical Research Sites
Track 11: Clinical Trials on different Diseases and Disorders
Clinical Trials for different diseases and disorders are conducted for evaluating one or more interventions (for example, drugs, medical devices, approaches to surgery or radiation therapy) for treating a disease, syndrome, or condition and also finding ways to prevent the initial development or recurrence of a disease or condition. These can include medicines, vaccines, or lifestyle changes, among other approaches.
Related Conferences:
7th Conference on Biomarkers, November 28-30, 2016 Baltimore, USA; Conference on Medical Ethics, June 09-11, 2016 London, UK; 6th Conference on Clinical Dermatology, May 05-07, 2016 Chicago, USA; 3rd Experts Meeting on Medical Case Reports, May 09-11, 2016 New Orleans, USA; 5th Clinical Trials Inspection Readiness Summit, August 08-09, 2016, Philadelphia, USA; ACRP Conference, April 29-May 2, 2017, Seattle, USA; FDA Clinical Trial Requirements Regulations, Compliance, GCP Conference, November 2-3, 2016 New Orleans, USA; Australasian Kidney, Trials Network, Chinese American Medical Society, Drug Development Institute, Indian Association for Statistics in Clinical Trials, Chinese Clinical Trial Register
Track 12: Medical Devices Clinical Trials
Medical device means any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use, software, material or other similar or related article, intended by the manufacturer to be used, alone or in combination, for human beings, for one or more of the specific medical purpose(s) of:
- Diagnosis, Prevention, Monitoring, Treatment or alleviation of disease,
- Diagnosis, Monitoring, Treatment, alleviation of or compensation for an injury,
- Investigation, Replacement, Modification, or support of the anatomy or of a physiological process,
- Supporting or sustaining life,
- Control of conception,
- Disinfection of Medical Devices
- Providing information by means of in vitro examination of specimens derived from the human body;
and does not achieve its primary intended action by pharmacological, immunological or metabolic means, in or on the human body, but which may be assisted in its intended function by such means.
Related Conferences:
Conference on Medical Devices, March 27-29, 2017 Orlando, USA; Conference on Therapeutic Drug Monitoring & Toxicogenomics, ,June 09-10, 2016 Dallas, USA; 10th Conference on Clinical Ophthalmology, November 28-30, 2016 Dubai, UAE; Conference on Clinical Laboratory Medicine, May 02-03, 2016 Chicago, USA; Conference on Legal Medical, June 09-11, 2016 London, UK; FDA Clinical Trial Requirements Regulations, Compliance, GCP Conference, November 2-3, 2016 New Orleans, USA; Outsourcing in Clinical Trials Nordics Conference 2016, September14-15, 2016, Copenhagen, Denmark; Mobile in Clinical Trials Conference, September 19, 2016 Boston, USA; ACCP 2016 Annual Meeting, September 25-27, 2016, Atlanta, USA; Association of Clinical Research Professionals, Society for Clinical Research Sites, American Association for Cancer Research, European Federation of Pharmaceutical Industries and Associations, American Lung Association
Track 13: Clinical Trials (Countries/Continents)
Clinical trials are research studies that explore whether a medical strategy, treatment, or device is safe and effective for humans. Study show which medical approaches work best for certain illnesses or groups of people. The purpose of clinical trials is research, so the studies follow strict scientific standards. These standards protect patients and help produce reliable study results. Clinical trials are one of the final stages of a long and careful research process. Clinical trials are conducted for every disease. The main reason for carrying out trials is to determine whether one treatment is better than another, 19% on average increase in number of registration per year in Clinical Trials, 47.0% increase in Market Investment in Clinical Trials from 2013-2015.
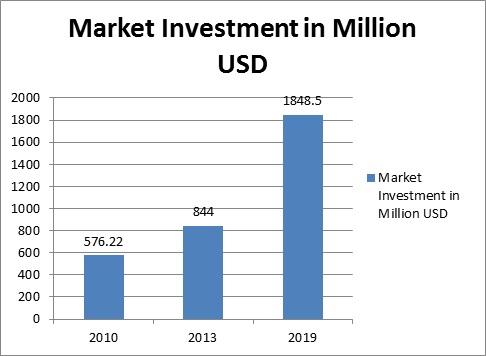
Clinical Trials Management System has made enormous progress in past few years, and according to Transparency Market Research the estimated net worth is approximately US$844 million in 2013, while growing at a compound annual growth rate of 14% during the forecast period, the worldwide Clinical Trials Management System market is expected to acquire market value worth US$1,848.5 million by 2019. United States is the major center in the field of Clinical Trials.
Related Conferences:
5th Conference on Translational Medicine, November 17-19, 2016 San Francisco, USA; 10th Conference on Clinical Ophthalmology, November 28-30, 2016 Dubai, UAE; Conference on Legal Medical, June 09-11, 2016 London, UK; Clinical Practice Compliance Conference, October 23-25, 2016, Phoenix, USA; Outsourcing in Clinical Trials Southern California 2016, October 26-27, 2016, California, USA; Exploratory Clinical Development World Europe Conference 2016, October 26-27, 2016, Berlin, Germany; Clinical Development Leaders Summit, November 01-03, 2016, Shanghai, China; FDA Clinical Trial Requirements Regulations, Compliance, GCP Conference, November 02-03, 2016, New Orleans, USA; Association of Clinical Research Professionals, Society for Clinical Research Sites, American Association for Cancer Research, Canadian Cancer Society
Track 14: Business Development in Clinical Trials
Business development in clinical trials aiming at developing and implementing growth opportunities within CRO’s, the basis for business development is successful Sponsor-CRO partnership and development of strategies to attract sponsors, pharmaceutical companies and nonprofit organizations.
Related Conferences:
2nd World Congress on Pharmacology, August 08-10, 2016 Birmingham, UK; Conference on Toxicogenomics, June 09-10, 2016 Dallas, USA; 3rd Experts Meeting on Medical Case Reports, May 09-11, 2016 New Orleans, USA; Conference on Legal Medical, June 09-11, 2016 London, UK; Preclinical and Translational Immuno-Oncology Conference, August 30-31, 2016, Boston, USA; Clinical Trials for Cancer Immunotherapy, September 1-2, 2016, Boston, USA; Clinical Practice Compliance Conference, October 23-25, 2016, Phoenix, USA; Outsourcing in Clinical Trials Southern California 2016, October 26-27, 2016, California, USA; Association of Clinical Research Organizations, American Society of Clinical Oncology, Association of Clinical Research Professionals, Society for Clinical Research Sites
Track 15: Clinical Trial Supply Management
The conduct of clinical trials is one of the most expensive aspects of the development of new medicinal products. It is important, therefore, that the studies should produce high-quality data in the shortest possible time. More companies are trying to use single, larger, complicated trials in an attempt to gain the greatest amount of information about a product and thus reduce the lead time to market. A key element in ensuring this goal is the close cooperation between those responsible for the provision of the clinical trial supplies. The provision of clinical trial supplies is usually organized by a special group, often within the product development department, and it is prudent to discuss a proposed trial with this group at an early stage so that any potential difficulties can be identified and resolved. The major steps in clinical trial supplies are 1)Placing an order for clinical trial supplies, 2) Manufacturing of clinical trial supplies, 3) Blinding of clinical trial supplies, 4) Obtaining comparators, 5) Packaging, 6) Labelling, 7) Documentation, 8) Expiry dating, 9) Dispatch of supplies, 10) Disposal of clinical trial supplies.
The value of some study drugs can reach tens of millions of dollars, making it essential to avoid overproduction, oversupply, and inventory expiration. With the high costs and strict handling requirements for many biopharmaceutical products entering clinical development, the logistics of clinical trial supplies are more critical than ever.
Related Conferences:
2nd World Congress on Pharmacology, August 08-10, 2016 Birmingham, UK; 7th Conference on Biomarkers, November 28-30, 2016 Baltimore, USA; Conference on Therapeutic Drug Monitoring , June 09-10, 2016 Dallas, USA; Preclinical and Translational Immuno-Oncology Conference, August 30-31, 2016, Boston, USA; Clinical Trials for Cancer Immunotherapy, September 1-2, 2016, Boston, USA; Summit for Clinical Ops Executives (SCOPE), January 24-26, 2017, Miami, USA; Clinical Data Analytics Conference, January 24-25, 2017 Miami, USA; Association of Clinical Research Professionals, Society for Clinical Research Sites, American Association for Cancer Research, Canadian Cancer Society, Association of Clinical Research Organizations
Track 16: Future of Clinical Trials
With the increasing challenges associated with bringing a medical product to market, and skyrocketing R&D costs, there has been a shift in biopharmaceutical commercialization and clinical trial strategies in order to minimize risk and maximize success. It is estimated that bringing medical products to market costs $1.3 billion and 1 out of every 5000 experimental compounds achieve FDA approval. Moreover, market saturation in many disease modalities is also forcing biopharmaceutical enterprises to focus their efforts on accessing patients with orphan/rare diseases.
The future of clinical trials illustrated as follows. Access and engage the patient online – Attract patients to the trial – Consent patients & convert to subjects – Remotely manage subjects & collect data. The sponsor plans to engage the patient through social media, such as Facebook, and regular e-mail updates in order to attract the patient to the trial, and once the patient agrees to learn more about the trial, the sponsor mails the patient a package containing mobile health devices, which collect medical diagnostic data and sends that data to the sponsor. The patient turns on the tablet, which contains an electronic consent and a video of a physician explaining the clinical trial in detail. Humanization in digital media is believed to be an effective tool that is used to communicate with patients. During the clinical trial, the subject is able to access live physicians either virtually or through nearby medical community centers. Remote nurses visit the subject at their homes to collect samples. In addition, the patient uses the mobile health device to automatically upload study data directly to the sponsor's EDC database.
Related Conferences:
2nd Conference on Clinical Trials, August 22-24, 2016 Philadelphia, USA; 5th Conference on Translational Medicine, November 17-19, 2016 San Francisco, USA; 7th Conference on Biomarkers, November 28-30, 2016 Baltimore, USA; 4th Conference on Clinical Pharmacy, October 31-November 01, 2016 Las Vegas, USA; Clinical Trial Forecasting and Budgeting Conference, January 24-25, 2017 Miami, USA; Clinical Technology and Innovation Conference, January 25-26, 2017 Miami, USA; Managing Outsourced Clinical Trials Conference, January 25-26, 2017 Miami, USA; Leveraging Real World Data for Clinical and Observational Research Conference, January 25-26, 2017, Miami, USA; American Society of Clinical Oncology, Leukemia & Lymphoma Society, Central Society for Clinical and Translational Research, ECFS-Clinical Trial Network- Europe, Australasian Kidney Trials Network
Track 17: Bioethics and Quality Regulation
Bioethics is the study of the typically controversial ethical issues emerging from new situations and possibilities brought about by advances in medicine. It is also moral discernment as it relates to medical policy, practice, and research. Bioethicists are concerned with the ethical questions that arise in the relationships among life sciences, biotechnology, medicine, clinical research, and philosophy etc. One of the first areas addressed by modern bioethicists was that of human experimentation. The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research was initially established in 1974 to identify the basic ethical principles that should underlie the conduct of biomedical and behavioral research involving human subjects.
Clinical research ethics are the set of relevant ethics considered in the conduct of a clinical trial in the field of clinical research. It borrows from the broader fields of research ethics and medical ethics. Quality of clinical trials depends on data integrity and subject protection. Globalization, outsourcing and increasing complexity of clinical trials have made the target of achieving global quality challenging. The quality, as judged by regulatory inspections of the investigator sites, sponsors/contract research organizations and Institutional Review Board, has been of concern to the US Food and Drug Administration, as there has been hardly any change in frequency and nature of common deficiencies. Good Clinical Practice (GCP) is the universal ethical and scientific quality standard for conducting clinical trials. The GCP standard applies to all aspects of the clinical trial process.
Related Conferences:
7th Conference on Biomarkers, November 28-30, 2016 Baltimore, USA; Conference on Medical Ethics, June 09-11, 2016 London, UK; 6th Conference on Clinical Dermatology, May 05-07, 2016 Chicago, USA; 3rd Experts Meeting on Medical Case Reports, May 09-11, 2016 New Orleans, USA; 5th Clinical Trials Inspection Readiness Summit, August 08-09, 2016, Philadelphia, USA; ACRP Conference, April 29-May 2, 2017, Seattle, USA; FDA Clinical Trial Requirements Regulations, Compliance, GCP Conference, November 2-3, 2016 New Orleans, USA; Australasian Kidney, Trials Network, Chinese American Medical Society, Drug Development Institute, Indian Association for Statistics in Clinical Trials, Chinese Clinical Trial Register
Track 18: Clinical and Medical Case Reports
A case report is a means of communicating something new that has been learnt from clinical practice. It could be about an unusual or previously unknown condition, a rare presentation or complication of a known disease, or even a new approach to managing a common condition. A case report provides the detailed report of symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports may contain a demographic profile of the patient and play major role in the field of medical research and evidenced based medicine. Moreover, case reports can serve as an early warning signal for the adverse effects of new medications, or the presentations of new and emerging diseases.
Related Conferences:
2nd World Congress on Pharmacology, August 08-10, 2016 Birmingham, UK; Conference on Therapeutic Drug Monitoring, June 09-10, 2016 Dallas, USA; 10th Conference on Experimental Ophthalmology, November 28-30, 2016 Dubai, UAE; Conference on Laboratory Medicine, May 02-03, 2016 Chicago, USA; Conference on Legal Medical, June 09-11, 2016 London, UK; FDA Clinical Trial Requirements Regulations, Compliance, GCP Conference, November 2-3, 2016 New Orleans, USA; Outsourcing in Clinical Trials Nordics Conference 2016, September14-15, 2016, Copenhagen, Denmark; Mobile in Clinical Trials Conference, September 19, 2016 Boston, USA; ACCP 2016 Annual Meeting, September 25-27, 2016, Atlanta, USA; Association of Clinical Research Professionals, Society for Clinical Research Sites, American Association for Cancer Research, European Federation of Pharmaceutical Industries and Associations, American Lung Association
Track 19: Pharmacovigilance and Drug Safety
The pharmacovigilance is related to collection, detection, assessment, monitoring, and prevention of adverse effects with pharmaceutical products, and it is needed in different stages of product life cycle, and the safety surveillance and risk management. Information received from patients and healthcare providers via pharmacovigilance agreements, plays a critical role in providing the data necessary for Pharmacovigilance to take place, in order to market or to test a pharmaceutical product, adverse event data must be submitted to the local drug regulatory authority. Finally pharmacovigilance is concerned with identifying the hazards associated with pharmaceutical products and with minimizing the risk of any harm that may come to patients by safety surveillance and risk management
Related Conferences:
6th Conference on Clinical Cardiology, November 14-16, 2015 San Francisco, USA; 7th Conference on Pharmacovigilance, September 19-21, 2016 Vienna, Austria; Conference on Legal Medical, June 09-11, 2016 London, UK; 3rd European Conference on Clinical Research, October, 17-18 2016, Prague, Czech Republic; 3rd Clinical Trials Phase I and Phase IIA Summit, October 18-19, 2016, Philadelphia, USA; Clinical Trial Supply East Coast Conference 2016, October 19-20, 2016, Princeton, USA; Outsourcing in Clinical Trials Canada Conference 2016, October 19-20, 2016, Vancouver, Canada; European Federation of Pharmaceutical Industries and Associations, American Lung Association, Association of Clinical Research Professionals, Society for Clinical Research Sites
Track 20: Entrepreneurs Investment Meet
2nd International Conference on Clinical Trials, scheduled during August 22-24, 2016 at Philadelphia, USA. This Clinical Trials Conference includes a wide range of Keynote presentations, Oral talks, Poster presentations, Symposia, Workshops, Exhibitions and Career development programs.
ConferenceSeries Ltd Organizes 3000+ Global Events Every Year across USA, Europe & Asia with support from 1000 more scientific societies and Publishes 700+ Open access journals which contains over 100000 eminent personalities, reputed scientists as editorial board and organizing committee members. The conference series website will provide you list and details about the conferences organize worldwide.
Clinical Trials Conference is one of the well-established conference among Pharmaceutical Conferences organized by ConferenceSeries Ltd
Clinical Trials Management System has made enormous progress in past few years, and according to Transparency Market Research the estimated net worth is approximately US$844 million in 2013, while growing at a compound annual growth rate of 14% during the forecast period, the worldwide Clinical Trials Management System market is expected to acquire market value worth US$1,848.5 million by 2019. United States is the major center in the field of Clinical Trials.
Why to attend???
Clinical Trials is a multidisciplinary program with broad participation with members from around the globe focused on learning about clinical research and its advances. This is your best opportunity to reach the largest assemblage of participants from Clinical Trials community that is from academia, clinical research entities, medical groups, related associations, societies and also from government agencies, pharmaceutical, biomedical and medical device industries.
Clinical Trials 2016 will discuss various disciplines involved in the pre-clinical research, conduct of clinical trials; it will educate health care researchers about design, operation, organizing, research computing, regulatory aspects and reporting of clinical trials. It promotes better understanding by the general public about the importance of clinical trials in prevention, diagnosis and treatment of disease.
Conduct presentations, distribute information, meet with current and potential scientists, make a splash with new clinical research developments, and receive name recognition at this 3-days event. World renowned speakers and the most recent techniques, developments, the newest updates in Clinical Research are hallmarks of this conference.
Who Should Attend
Directors/Senior Directors/Executive Directors and Vice Presidents/Senior Vice Presidents/Executive Vice Presidents and Heads/Leaders/Partners of
- CROs and CMOs
- Clinical Research Sites
- Pharma/Biotech and Medical Device industries
- Hospitals, Associations
Medical Directors, Principal Investigators, Methodologists, and other clinical research professionals along with Academicians: University Faculties like Directors, Senior Professors/Assistant Professors/ Associate Professor, Research Scholars, scientists who are related to clinical and medical research.
Clinical/Pharmaceutical and biotech industry professionals with responsibilities in:
- Clinical Research & Development
- Clinical Design/ Protocol Design/ Clinical Strategy
- Global Clinical Operations/ Clinical Outsourcing
- Biostatistics/Data management
- Patient Recruitment/Enrollment
- Clinical Trial Management/Clinical Trial Supplies
- Regulatory Affairs
Importance and Scope of Conference
Clinical trials are research studies that explore whether a medical strategy, treatment, or device is safe and effective for humans. Study show which medical approaches work best for certain illnesses or groups of people. The purpose of clinical trials is research, so the studies follow strict scientific standards. These standards protect patients and help produce reliable study results. Clinical trials are one of the final stages of a long and careful research process. Clinical trials are conducted for every disease. The main reason for carrying out trials is to determine whether one treatment is better than another, 19% on average increase in number of registration per year in Clinical Trials, 47.0% increase in Market Investment in Clinical Trials from 2013-2015
Clinical Trials Management System has made enormous progress in past few years, and according to Transparency Market Research the estimated net worth is approximately US$844 million in 2013, while growing at a compound annual growth rate of 14% during the forecast period, the worldwide Clinical Trials Management System market is expected to acquire market value worth US$1,848.5 million by 2019. United States is the major center in the field of Clinical Trials.
Clinical Trials 2016 Conference will provide platform for the Clinical Research Organizations and Pharmaceutical Industries to share about the efficacy of their products and new technologies. This conference provides the information about new drugs and therapeutic techniques to healthcare professionals.
Conference Highlights
- Pre-Clinical Research
- Conducts of Clinical Trials
- Clinical Study Designs
- Innovations in Clinical Trials
- Clinical Data Management and Statistics
- Transforming Trial Methodologies
- Clinical Trials in Oncology
- HIV/AIDS Clinical Trials
- Diabetic Clinical Trials
- Cardiovascular Clinical Trials
- Clinical Trials on different Diseases and Disorders
- Medical Devices Clinical Trials
- Clinical Trials (Countries/Continents)
- CRO Clinical Trials
- Clinical Trial Supply Management
- Future of Clinical Trials
- Bioethics and Quality Regulation
- Clinical and Medical Case Reports
- Pharmacovigilance and Drug Safety
- Entrepreneurs Investment Meet
Statistics of Clinical Trials Study
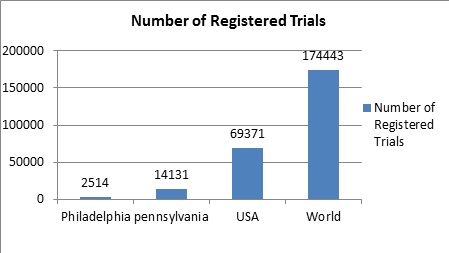
Market Share
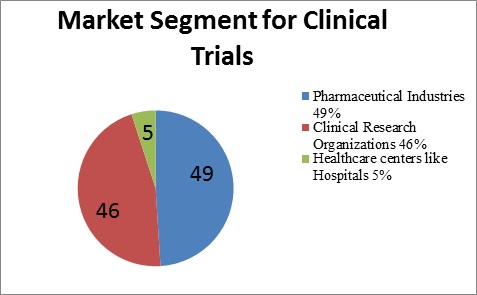
Market Statistics
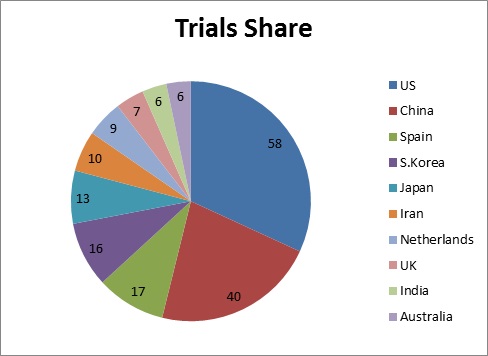
Global Market Share
Investment in year 2015
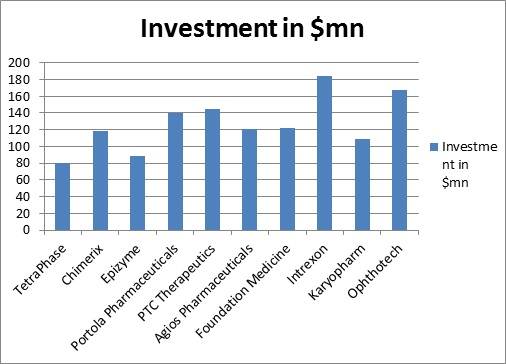
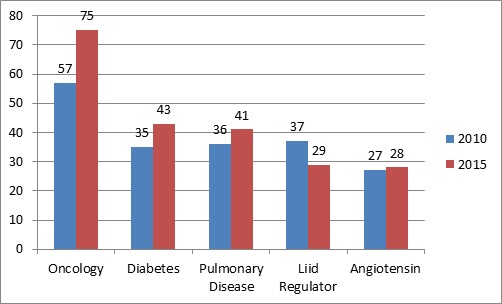
Area wise forecast for Spending in 2010 and 2015
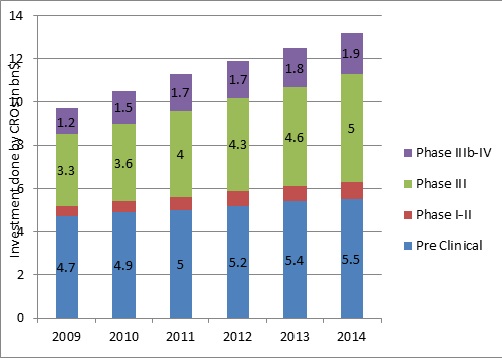
Market Analysis
Maximum investment have been done for Pre-Clinical research and Phase II-IV of the Clinical Trials, while least is done for Phase I and Central Lab
Pre-Clinical, Phase I and Phase II costs $120,000
Phase III Clinical Trials cost - $75000
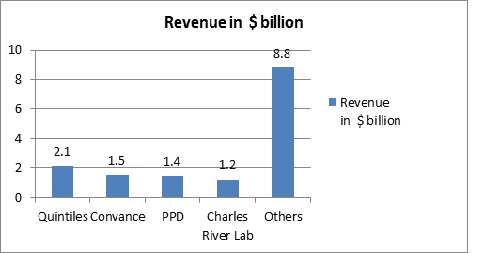
Few Majors in Clinical Trials
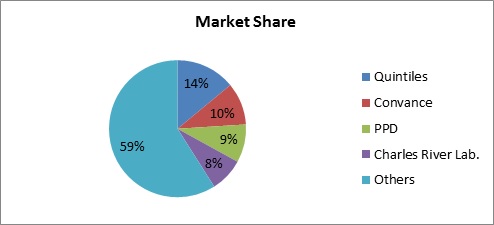
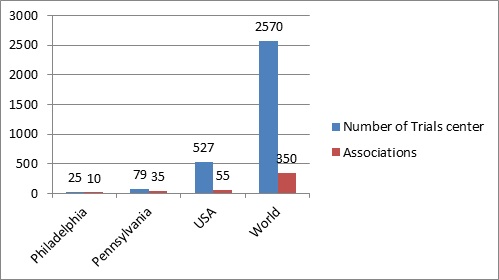
Clinical Research Institutes, Hospitals & Associations
Societies and Associations in USA
- American Association for Cancer Research
- Canadian Cancer Society
- Association of Clinical Research Organizations
- American Society of Clinical Oncology
- Leukemia & Lymphoma Society
- Central Society for Clinical and Translational Research
Societies Worldwide
- ECFS-Clinical Trial Network- Europe
- Australasian Kidney Trials Network
- Chinese American Medical Society
- International Drug Development Institute , Belgium
- Chinese Clinical Trial Register, China
- Australian Clinical Trials
Conference Highlights
- Conducts of Clinical Trials
- Pre-Clinical Research
- Clinical Study Designs
- Innovations in Clinical Trials
- Clinical Data Management and Statistics
- Clinical Trials on different Diseases and Medical Devices
- Clinical Trials (Countries/Continents)
- Clinical Trial Supply Management
- CRO/ Sponsorship and Future of Clinical Trials
- Bioethics, Quality Regulation and Case Reports
- Pharmacokinetics and Pharmacodynamics
- Therapeutic Drug Monitoring and Drug Quantification
- Pharmacovigilance and Drug Safety
- Entrepreneurs Investment Meet
- Clinical Research and Trials on Diabetes / Cancer / AIDS
- Toxicogenomics Challenges and Applications
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | August 22-24, 2016 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | Day 3 |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Clinical Trials
- Journal of Clinical Case Reports
- Journal of Clinical Research and Bioethics
Abstracts will be provided with Digital Object Identifier by


























